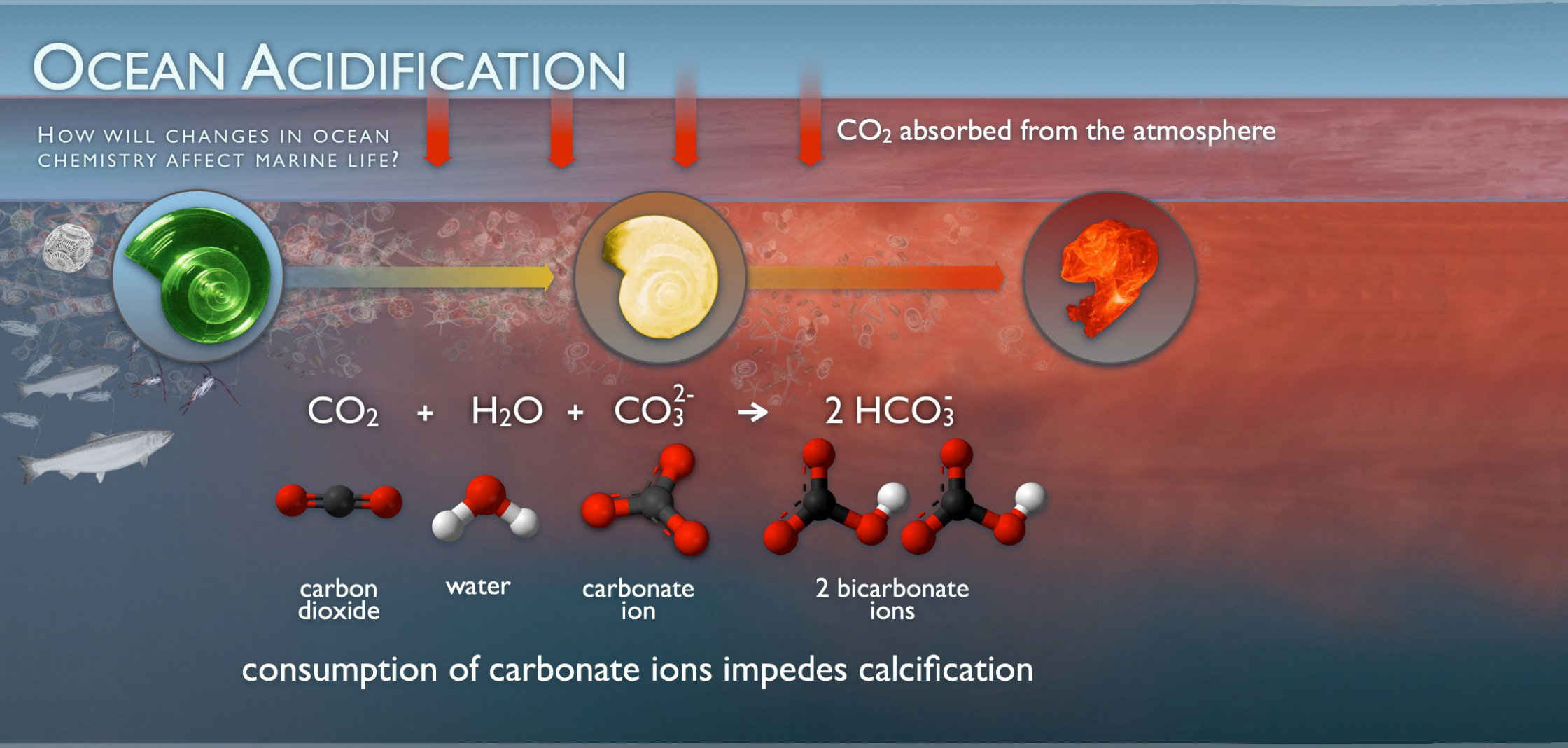
What is Ocean Acidification?
While carbon dioxide occurs naturally in the environment, since the Industrial Revolution began in the late 1700s, humans have been burning greater and greater amounts of fossil fuels like coal, natural gas and oil. Today, the amount of carbon dioxide in our atmosphere is estimated to be highest it has ever been in the last 800,000 years of Earth’s history.
Before the industrial revolution, the amount of carbon dioxide in our oceans was about 280 parts per million. Today, it’s closer to 400 ppm — and that number is increasing.
All of that excess carbon dioxide has to go somewhere.
While some of it is absorbed by plants and trees, worldwide deforestation has reduced the amount of carbon dioxide that our forests can absorb. The world’s oceans are picking up the slack.
As a gas, carbon dioxide dissolves in water. (Think of the bubbles in your fizzy water.) When carbon dioxide dissolves in seawater, its molecules react with seawater, forming carbonic acid, which then transforms into bicarbonate and carbonate ions.
While each of these forms of carbon exist naturally in seawater, as the oceans absorb excess carbon dioxide, the balance shifts and the carbonate is transformed to bicarbonate — changing the pH of the ocean and making it more acidic.














